Three Different Phases of Powders
Powders are a unique three-phase material. It consists of a solid phase in the form of particles, a gas phase between the particles, and a liquid phase on the surface of the particles or inside their structure.
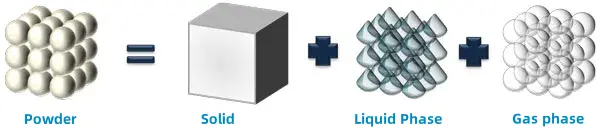
A powder is a loose collection of solid, liquid, and gas phases. There is a common misconception that the behavior of a powder can be described simply by understanding its flow properties. These properties are discrete, quantifiable with a single number. Unfortunately, both of these ideas are incorrect. This is why, even in the 21st century, we still do not fully understand the behavior of powders.
Imagine a glass jar containing loose powder. Think about how the powder will behave if the jar is knocked over. Consider how the powder will behave differently if the jar is picked up and repeatedly tapped on a hard surface. Any difference in the behavior of a powder in its loose state versus its compacted state is due to its properties. For example, if the powder is like dry sand, its behavior may be roughly the same before and after compaction. However, if the powder is like flour, very different flow properties can be observed after compaction. This is an important and typical property of powders.

While these properties are crucial to understanding powder behavior, external variables—such as the degree of aeration or consolidation—are equally important. The chemical and physical properties of the particles do not change in these states. It’s the amount of air and the contact pressure between the particles that causes the flow to differ significantly.
Influence of External Variables
As shown in the previous examples, powders behave very differently when they are aerated, loose, or consolidated. Some powders are very sensitive to these variables, while others are not. Some powders may flow well when aerated and loose, but cause trouble when consolidated (such as toner). Other powders may have reasonable (good) flow when loose, and not much effect when consolidated, but have a real improvement in flow when aerated (such as ceramic powders, see video). Based on these observations, it is unlikely that a single number will adequately describe how a powder will react to large amounts of aeration or high levels of consolidation during processing and application.
In terms of flow properties, consolidation stress and air content are the two variables that have the greatest influence. However, powder behavior is also affected by processing speeds, such as mixing speed or line filling speed, as well as other factors like ambient humidity levels and storage time. A powder with excellent properties may exhibit poor performance when stored or processed in an environment with humidity slightly higher than normal.
Powder behavior
| External variables | When and where they occur | Effect |
| Consolidation | Vibration / Slap Direct Pressure (hoppers, IBCs, kegs) | Increased pressure, contact area, and number of contact points between particles Reduced air content between particles (decreased porosity) |
| Aeration | Gravity unloading, mixing, pneumatic conveying, atomization | The pressure, contact area and number of contact points between particles decrease. The air content between particles increases (increased porosity). |
| Flow (shear) rate | In the powder, Between the powder and the equipment wall, Mixing | Mainly non-Newtonian flow Increased resistance to flow at low flow rates |
| Humidity | Storage Processing Human addition (granulation) | Increase the adhesion between particles Increase the adhesion between particles Increase the conductivity |
| Static electricity | Discharging from hopper Pneumatic conveying High shear mixing | Increase the bonding strength between particles Powder adheres to the equipment |
| Storage time | Raw materials/intermediate materials | Consolidation, agglomeration, permanent impact on downstream performance |
Other situations for particle properties
While it may be relatively simple to control some variables, changes in air content and consolidation stress during processing are often difficult to avoid. Even as powders pass through a basic conveyor chute, air can be introduced, and consolidation can occur. Powders expand and the particles become more spaced apart when aerated. This happens in many processes, including mixing, filling, and discharging operations. Even when pneumatic conveying without an external air supply is not used.
Recognizing that any of these external variables can alter powder behavior is the first step toward better understanding process performance. Next, measuring the powder’s response to various external variables can provide insight into why the powder behaves in a particular way and present opportunities to optimize both formulation and production efficiency.
Influence of Particle Properties
In addition to the external variables, there are many more particle properties that affect the behavior of loose powders. Particles are complex, and there are far too many parameters to fully describe them. Particle size and size distribution are often considered, and these two parameters are still important.
However, there are many other particle properties that influence the overall behavior of the powder. Key particle properties include:

Note: Each of these properties is more of a distribution than a single value. Some can be measured directly, while others are more difficult to quantify. However, all of them can impact the way a powder behaves. Given the complexity and variety of particle properties and external variables, predicting powder behavior using basic mathematical methods is extremely difficult, if not beyond our current capabilities.